Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive B-cell lymphoma with high clinical and biological heterogeneity. Despite current effective immunochemotherapy, up to 40% of patients do not respond or develop refractory disease. DLBCL is characterized by two major cell-of-origin (COO) subtypes, germinal center B cell-like (GCB) and activated B cell-like (ABC), with recent research uncovering additional molecular subgroups based on genomic alterations or tumor microenvironment (TME) features. However, current classifiers use bulk or deconvolution profiling which blurs intra-tumor heterogeneity, hindering the discovery of true functional cell states underlying clinical diversity. Here, we constructed a multimodal single-cell atlas of 62 DLBCL cases, integrating single-cell transcriptomes, immune repertoire and genetic features, to decrypt recurrent B cell states and TME ecosystems while identifying clinically relevant prognostic biomarkers (Fig.1).
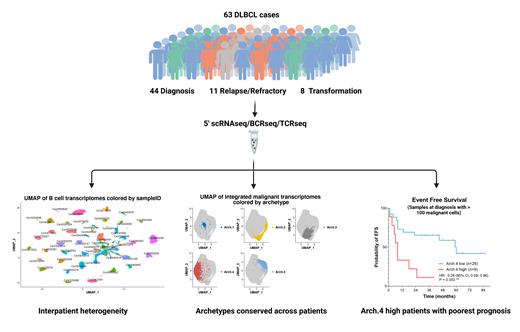
We performed simultaneous 5'-end single-cell RNA, B-cell receptor (BCR) and T-cell receptor sequencing on 63 DLBCL lymph node biopsies, recovering 109,294 cells after stringent quality controls. These biopsies were obtained from the real-world, clinically-annotated, multicentric CeVi collection and included 43 patient samples at diagnosis (ABC = 17, GCB = 11, Unclassified = 6, Not Evaluated = 9; 21 of which did not achieve event-free survival at 24 months (EFS24) after R-chemotherapy regimens), 11 relapsed/refractory DLBCL and 8 DLBCL transformed from indolent lymphoma. We distinguished malignant B-cells from TME using canonical markers and BCR sequence, exploring heterogeneity in both components. We developed an original bioinformatic approach to define malignant B cell transcriptional archetypes shared across patients. To assess whether subclonal genetics are associated with transcriptional states, we inferred copy number variations (CNV) and studied BCR intraclonal variations of malignant cells. We also integrated TME data (39,674 cells) to quantify subpopulations and clonally expanded T-cell subsets, and performed robust statistical analyses to identify clinically relevant features linked to patient outcomes.
To circumvent interpatient heterogeneity in malignant B cells, we defined transcriptional cellular archetypes conserved across patients by combining 3 unsupervised methods: rank-biased overlap meta-clustering, variational autoencoder and canonical correlation analysis integration. We identified 5 cellular archetypes (Arch.1-5): Arch.1 was characterized by plasma cell identity, Arch.2 by MYC and NFKB pathways, Arch.3 by MYC pathway only, Arch.4 by quiescence markers, and Arch.5 by GC markers. Several archetypes co-existed in each tumor sample, and archetype identity was superimposed to the transcriptional profile conferred by COO molecular class as an additional layer of transcriptional heterogeneity. Cell state-specific CNV and BCR subclones were very rare, suggesting that subclonal genetic changes were not the main drivers of malignant B cell plasticity and archetypes. Kaplan-Meier analysis showed a significant negative association between proportion of Arch.4 cells and continuous EFS in our diagnosis cohort. Unsupervised analysis of archetype composition further distinguished ABC patients who achieved EFS24 (enriched in Arch.3 cells) from those with a particularly poor prognosis (enriched in Arch.4 cells). Integrated analysis of the TME revealed three major ecosystems, including one enriched for effector and cytotoxic T cells. In diagnosis samples, pseudo-temporal trajectory analysis revealed the enrichment of clonally-expanded hyperactivated CD8+ T cells expressing canonical exhaustion markers in patients achieving EFS24, suggesting a positive link between the extent of CD8+ T cell differentiation and response to R-CHOP based regimens.
Shedding new light on DLBCL biological and clinical heterogeneity, our comprehensive single-cell atlas of DLBCL uncovered recurrent tumor cell states and TME features crucial for stratifying non-responsive ABC patients and guiding treatment decisions. Ongoing single-cell spatial transcriptomics analyses will be used to further validate our findings and elucidate archetype-TME interactions that may drive malignant B cell plasticity, with the potential to uncover novel therapeutic targets.
Disclosures
Gaulard:Sanofi: Research Funding; Roche: Other: travel and congress support; Takeda: Consultancy. Jardin:Janssen, Gilead, AbbVie, F. Hoffmann-La Roche Ltd, BMS, Takeda: Honoraria. Brisou:Novartis: Consultancy. Laurent:F. Hoffmann-La Roche AG: Research Funding; Janssen Pharmaceuticals: Honoraria. Huang:BMS: Current Employment. Stokes:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Seth:BMS: Current Employment. Ortiz:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gandhi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Nadel:BMS: Research Funding; diatech pharmacogenetics: Consultancy, Honoraria. Milpied:BMS: Research Funding. Roulland:BMS: Consultancy, Other: Travel Support, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal